Posted on January 19, 2011 by damirl
Yiling’s pNIC-Bsa4 + PTP1B + KAN was expressed today and is currently stored in the -20 fridge on the second shelf in a 50 ml conical tube. The side is labeled with all the necessary info and has Yiling’s initials on it (YW).
The dry pellet weight was determined to be ~1.6g.
Good luck with purification! (I’m crossing my fingers for a good yield).
Filed under: Mentors | Leave a comment »
Posted on November 28, 2010 by damirl
I hope everyone is having a wonderful break and is excited (much like I am) to get this last week done with. I’m sure many of you will be taking tests like I will, so good luck! As for VDS, we’re getting to the end. This past week (er, two days really) I managed to finish my DMSO tolerance assays and both of the trials for my inhibitor. Sadly, it did not seem to work 😦 Tell me what you guys think – an image of all my data is below. I also worked on my notebook for the final check and managed to analyze my data some in between bites of turkey. As for this last week, I’ll be testing another inhibitor (I’ll work out a comparative swap with one of you) and then focusing on getting my notebook together for Friday. Today, I’ll also be working on an updated version of my M&M for Dr.B that I will email to him and put up on Google Docs for you all to take a look at. I’ll see everyone in lab – best of luck in the push for home!
Oh and in case anyone was wondering, the CA7 Km that I reasoned to use was 3 (since there wasn’t an exact one on BRENDA).
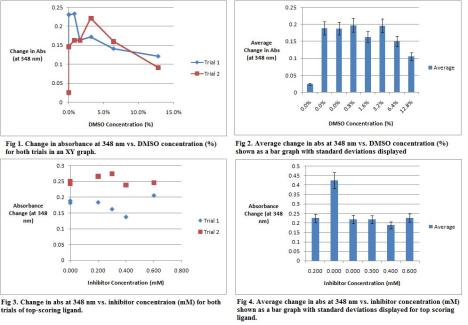
DMSO Tolerance and Inhibitor 1 Data
Filed under: Uncategorized | 2 Comments »
Posted on November 22, 2010 by damirl
Hey everyone! Happy (early) Thanksgiving! Sorry for the slightly late post – I had a computer error last night when trying to get my update online. This week was a productive one and is leading up to the work that will be done the upcoming week, inhibition assays. For the past week I did 2 enzyme varying assays (1 with my protein and the other with the stock). Although they were both good, I decided to stick with the stock for good measure and because it was slightly more effective. I also did both substrate varying assays and 1 DMSO assay. I collected the data for all of these and have included some images to let you all know where I stand. I also spent a lot of time last week working on my journal club presentation and my notebook. This week, I plan to finish my other DMSO assay and analyze the results, show them all to Dr. B, and proceed to inhibition assays. I will also be revising my Materials and Methods to give to Dr. B tomorrow and continue work with my lab notebook for the final check. I hope everyone has a successful week and I’ll see you in lab very shortly.
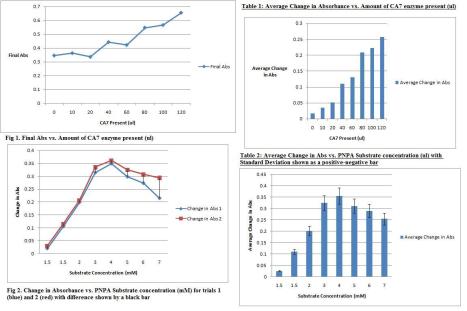
Varying Enzyme/Varying PNPA Results
Filed under: Uncategorized | 1 Comment »
Posted on November 14, 2010 by damirl
Hey everyone! I hope you all had a good weekend while I was home in Houston. This week was sort of a transition period for me. I spent the early part of the week concentrating my CA7 protein down to 1 ml and Nanodropping my 2 .5 ml samples – they turned out to be around 2.5 mg/ml and 3 mg/ml (can’t remember exact value because Dr. B has out lab notebooks). I input the values into the Google spreadsheet, if you want to take a look, and went on perform the calculations for my assays (prep of stock enzyme). I also worked on the Materials and Methods section of my paper and turned it in on Tuesday to Dr. B for review. Instead of just including cloning, however, I did the materials and methods for all the parts (up to inhibitor assays) so I could be a little ahead of the game for my paper. I spent the middle to latter part of the week working mostly on my notebook and starting my journal club presentation for this week. Additionally, I completed the data collection portion of Dr. Monzingo’s workshop and got to learn how to loop a crystal and set it up for diffraction. We also worked out indexing diffraction patterns for future analysis. As for the upcoming week before turkey day week, I plan to do both my enzyme and substrate assays (tomorrow most likely) and get ready to do inhibitor assays with my top-ranking compound when it arrives. I also may test other inhibitors which we already have available, if time permits. I will also be participating in the last portion of Dr. Monzingo’s workshop – structure determination, beginning tomorrow. I am very excited for this part and can’t wait to see how initial diffraction data is transferred into a three-dimensional structure. Good luck to everyone and let’s have a productive week!
Filed under: Uncategorized | Leave a comment »
Posted on November 7, 2010 by damirl
Hey everyone! I hope your weekend has gone well and that you’re all ready for another week of lab progress. This past week I spent a lot of time, as I’m sure many of you did, working on the aggregate run and consolidating my final results for Dr. B. Also, I purified and characterized my protein and am ready to move on to the next step! Dr. B told me to concentrate my protein early this coming week, run a sample test assay and start some enzyme assays. He also told me that he plans to order my top compound, which is surprisingly cheap. I am very excited for this because the compound is predicted to have a good deal of inhibition as well as a very low logP value (.45) and it manages to fit into all of Lipinski’s rules pretty well. So, I am looking forward to a week filled with the aforementioned. Additionally, I will be working on updating my notebook with the results of my individual library runs for Friday’s notebook check and Dr. Monzingo has decided to offer a crystal data workshop regarding x-ray diffraction and cryocrystallography which I fully plan to participate in. If you would too, just let me know and I’ll get you the information for the meeting on Monday. Best of luck everyone and let’s have a progressive week, make some real headway, and cure cancer.

Elution 1 Results obtained via Nanodrop protein A280 setting on 11-2
Filed under: Uncategorized | 2 Comments »
Posted on October 31, 2010 by damirl
Hey VDS-ers,
I know this doesn’t really belong up here but its a good cause so I figured it would be worth posting in case some of you are interested. I ran the Race for the Water this morning and it was a blast and, with the great weather nowadays, it’s the perfect time to be outdoors. I’ll be running the Race for the Cure next weekend, Nov. 7 at around 7 am and I’d love to see some VDS-ers down there. It’s a great cause (who doesn’t want to stop breast cancer?), awesome exercise, and you get a shirt! So check out the website below and I’ll see you come race time!
Race for the Cure
Filed under: Uncategorized | 3 Comments »
Posted on October 31, 2010 by damirl
Hey everyone,
This week I spent a bunch of time working with some of you to perform a group protein expression. I will be purifying on Monday and checking my elutions and will hopefully see a good yield in there (fingers crossed). I also used the Phoenix crystallization robot on Tuesday to crystallize some protein which I will review this Tuesday at Dr. Monzingo’s lab. I’ve also been screening a lot and getting my results together (8 libraries, including 3 “big” ones) and began my aggregate screen earlier today. I am excited for my results since I’ve had about 8-9 ligands with a gold score over 70, including one that was around 82! I’ll be evaluating my results later this week and sending them to Dr. B to get started on some assays in the near future. I was also at open house with many of you but, sadly, didn’t get to talk to anyone. This was probably due to my early shift, however. As stated, this week I’ll mainly be working on purification and characterization, alongside continuing my aggregate screen in preparation for the coming weeks. Also, I’ll finish up the crystallization workshop and let you guys know what it was like. Happy Halloween everyone and I’ll see you all in the lab!
Filed under: Uncategorized | Leave a comment »
Posted on October 24, 2010 by damirl
Hey everyone,
This week I spent the bulk of my time expressing CA7 protein for the second time this semester in hopes of getting it to work. Sadly, I do not think it did. I obtained an elution 1 concentration of .18 mg/ml and an elution 2 concentration of .11 mg/ml. I’m not sure what is happening in the purification step of the process, but I am pretty sure that is where the error is coming from. I will ask Dr. B for feedback when I show him my results tomorrow and hopefully I can get this to work out. Also, during the course of this past week, I stopped screening the huge library Chembridge-diversity3D because it was being screened very slowly and performed cluster clean-up. I then began screening the same library again and am waiting for it to finish. I also screened both runs of ION_Channel3D and analyzed the results only to have obtained no compounds worth investigating. In addition to the library I am still screening, I also began screening the MolecularWeightSet library in hopes of getting some good hits. This week I will continue my screening (probably start another library) and figure out what to do about my expression (either run a gel to see where the protein was lost or perhaps even start all over again (I hope not)). Also, I’ll be going to the crystallization workshop on Monday to develop some of the skills that I’ve only read about in journal club and preparing for the open house this Thursday by designing my slides for the communal PowerPoint. Good luck everyone and I’ll see you in lab!
Filed under: Uncategorized | Leave a comment »
Posted on October 19, 2010 by damirl
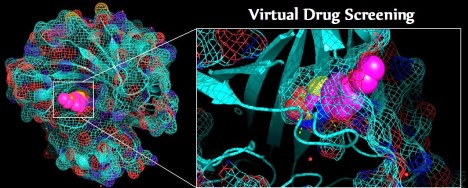
CA7 shown as a mesh-cartoon hybrid, colored by element, with EZL substrate binding into the active site as spheres. The right features a zoomed-in binding view of EZL sagittally bound in the active site with polar contacts shown as dashed lines.
Click here to see real size
Filed under: PyMol Image Contest | 1 Comment »
Posted on October 17, 2010 by damirl
Hey everyone! This week I spent a good deal of time making and running an SDS gel to the results of my expression. Last week, I noticed that my concentration was low and that some of the protein had passed into my second elution. Accordingly, I conducted a gel check to be sure and, sure enough, a great chunk of my protein was lost during the wash phase of purification (although the expression hadn’t produced much protein anyway). As a result of this, it looks like I’ll be expressing again this week. I also spent a big part of the week working on my lab notebook and analyzing my first two screening results. In terms of potential inhibitors, there was only one compound that seems to be worth testing, the top-ranking compound from the H9 library. It was the only result that had a GOLD score over 70 (around 70.5). I will ask Dr. B if it is a worthy compound for in vitro testing and proceed from there. Also, I am in the process of screening my third library, the ChemBridge diversity 3d set. It is the biggest library I have screened thus far (around 45,000 compounds) so hopefully I’ll get some good results. Other than that, expression will dominate my schedule but hopefully it will produce some good protein to run assays with! Good luck everyone and see you in lab.
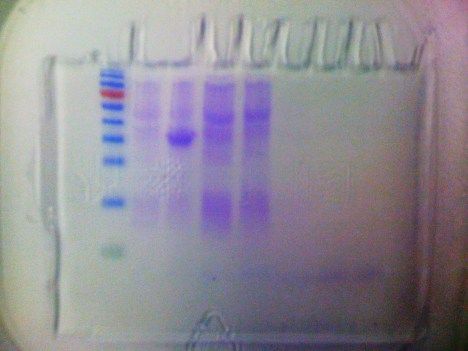
SDS gel for CA7 expression on 10/14 Lanes: skip, ladder, sample 0, sample 1, sample 2, sample 3, sample 4, sample 5, sample 6, skip
Filed under: Uncategorized | 2 Comments »
Posted on October 10, 2010 by damirl
Hey everyone,
This week I spent most of my time in the lab expressing and purifying CA7. Sadly, it doesn’t seem like it was all that successful. Attached you can see the Nanodrop readings for my two elutions. I am going to run a gel in the early part of this week to see if I can figure out what happened and hopefully avoid having to spend another week expressing. Also, I spent a large part of the week, as I’m sure many of you did, working on the research report due today. I have just uploaded it to GoogleDocs so take a look and tell me what you think! Further, I analyzed my results from my first virtual screen this week and am in the process of putting them together in an organized, coherent way to share with you. I began my second screen (this time with the KINASet3d library) and will get those results up as soon as they are done. During the course of this week I plan to run my gel and figure out what to do about my protein expression (either consolidate the 2 elutions or re-express) and continue my second virtual screen. I will also work on my lab notebook in preparation for this week’s check and attempt to post comments on here for often. Have a great week fellow VDS-ers!
-
-
Elution 1 Nanodrop results (Protein A280)
-

-
Elution 2 Nanodrop results (Protein A280)
Filed under: Uncategorized | 1 Comment »
Posted on October 3, 2010 by damirl
Hey everyone!
This week I was supposed to be working on my scaled up protein expression but, sadly, it didn’t work out. Everything began normally on Monday and I started my overnight culture but then Tuesday’s unforeseen events overtook us all and my expression was lost. Instead of mourning over my lost colonies, however, I decided to postpone the expression until this week and begin on the virtual screening of my target, CA7. I begin on Wednesday and the first run lasted until Friday night. I just started my second run from home and am excited for the results! I’ve included an image of my bestranking.lst file from my first run, displaying the top-ranking compounds. I also spent time working on the final draft of my report and preparing for the coming week. This week I will continue the virtual screening of my target (and hopefully get some great results), work on my report, and, of course, perform that ominous goliath known as scaled-up expression. I hope everyone has a great week and good luck in the lab. See you there!
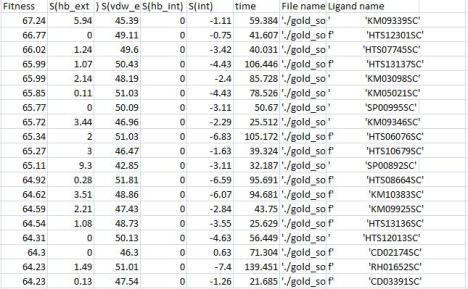
Fig 1. Excel results of bestranking.lst file after first run with CA7 (H9 library)
Filed under: Uncategorized | Tagged: CA7, Damir, Ljuboja, update, week 4, Weekly | Leave a comment »
Posted on September 26, 2010 by damirl
Hey everyone! This week I began my protein expression of CA7. I transformed the plasmid in BL21 (DE3) Novagen cells and grew up some protein on Kan/LB plates. I then did a little test run and performed an overnight transformation for an extended period of time (5pm one day to 1 pm the next) and checked the OD(600) and found it to be .17. This was done as an estimate in order to orient my scheduling for the coming week, which will be very busy. Furthermore, I spent a lot of the week working on the rough draft of my target research report (which I JUST sent to Dr. B). I found it to be quite difficult to locate some of the information, particularly in regards to the proposed methods. Did anyone else have this problem? As for the coming week, I will be working on finishing up my research report and taking it from a rough to a final draft. Primarily, though, I’ll be doing a scaled up expression of CA7 and purifying it (and characterizing, if I can get to it). Good luck and I’ll see you all in lab!
Filed under: Uncategorized | Leave a comment »
Posted on September 18, 2010 by damirl
Hey everyone. For me, the early part of this week was oriented around the two refreshers. By Wednesday, I managed to finish both of them and upload my results to GoogleDocs for you all to see. During the latter part of the week, I spent time organizing my lab notebook for Friday’s check, preparing and sending off my Midiprep results to be sequenced, and analyzing the results. I just finished comparing and BLAST-ing my results today so I’ll be uploading those to GoogleDocs this weekend as well. As for next week, I’ll be focusing on two major tasks – The first is the target research report, which I hope to beging very early on (if someone knows where the instructions/guidelines sheet is, please let me know!). The second is expressing and purifying my pNIC-Bsa4 + CA7 protein. I hope to finish this by the end of the week and finally be ready to go with some virtual screening and (hopefully) find some good inhibitors. I hope everyone has a great week ahead of them and I look forward to seeing you all in the lab!
Filed under: Uncategorized | Tagged: 3, Damir, Ljuboja, update, week, week 3 update | Leave a comment »
Posted on September 12, 2010 by damirl
Hello everyone! This week was much more progressive for me than the last. I managed to finish my Midiprep by Wednesday and nanodropped it (twice) to get the concentration – 232.0 ng/ul (I have 100 ul of total volume). I then split this pNIC-Bsa4 with CA7 into two tubes, one of which I put in the viral evolution freezer and the other into the -20 degree Celsius fridge. During Wednesday afternoon and Thursday I did the Pymol refresher in the computer lab and completed the actual protocol but I still need to complete the mini-write up associated with it (which I plan to do today). Furthermore, in regards to the virtual refresher, I have performed my first run and plan to start the second today in hopes of wrapping that up before the meeting on Tuesday. When both are completed I will send them over to Dr.B in addition to uploading the requested parts onto GoogleDocs for you all to see. By Tuesday or Wednesday I plan to submit a sample of from my Midiprep results to be sequenced at the DNA core and hope to analyze them by the end of the week. Also, near the end of the week I will be organizing/updating my lab notebook with the past week’s work and hopefully be near starting my initial virtual screen (yay!) or perhaps performing an enzyme assay on my pNIC-Bsa4 +CA7. Good luck to everyone and I hope we all have a great week ahead of us!
Filed under: Week 2 update | Leave a comment »










You must be logged in to post a comment.