
Fig. 1- DNA Concentration of 33.3 ng/ul and the DNA grown up for this Nanodrop was from the bacteria growth in the 15ml tubes which cut the Oxygen supply.
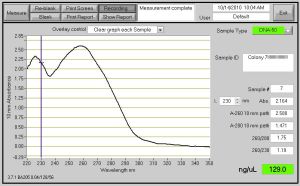
Fig. 2- DNA Concentration of 129 ng/ul and the DNA grown up for this Nanodrop was from the bacteria growth in the 14ml round bottom tubes which does not cut the Oxygen supply. Also, when spinning down the cells for this DNA, I did not take out the toothpicks.
I have 16 pictures from Nanodrop after I transformed, grew up the bacteria and Miniprepped them. The top 2 are posted, because I figured you would not want to see 16 pictures. After the Masterplate grew up, I took a tooth pick, and sequentially added each numbered bacteria colony to a numbered tube. Each Numbered tube had 5ml of LB with 50 ug/ml Kanaymycin (5 ul of Kanamycin per tube or 250 ng for all the 8 tubes.) I did not spilt up my bacteria growth and grew them up for 8 hours straight. After my bacteria grew, I took out the toothpicks and spun them down at 5000 rpm for 5 min. Next, Mini-Prep was performed on the DNA and then came the fun part. After mini-prep (I eluted in 50 ul), I proceeded to the dreadful Nanodrop. The highest DNA Concentration for the first time was 33.5 ng/ul. So, guess what, I had to perform these steps all over again. Although, I had to repeat some steps were changed along the way. When I was growing up my bacteria, I used a round bottom tube this time instead of a 15 ml colonial tube. The round bottom tubes are better because they have a loose top which enable the bacteria to consume more Oxygen for growth and the round bottom tubes are much CHEAPER than the other tubes. When spinning down the round bottom tubes, I had to parafilm the tops of them, so the liquid was secure in the tube. The first time I spun down in the 15ml tubes, I took out the toothpicks, but the second time I did not. Nothing was effected. Miniprep was done next, and the first time I eluted in 50 ul ph 8.0 water, but the second time I eluted in 30 ul of Tris-HCl. On average, my concentration of DNA from the first Miniprep was 26 ng/ul and for the second Mini-Prep it was about 52 ng/ul. In conclusion, the second time I minipreped with less elute I got a stronger concentration of DNA for use of RE Digest and DNA Sequencing. Also, for RE Digest the enzymes I will be using are SspI and BsrgI.
Filed under: cloning | Tagged: clones, colonies | Leave a comment »



You must be logged in to post a comment.